New Cable Enables EVs to Charge Within Five Minutes
NEWS

A new cable (undergoing tests above) could reduce an electric vehicle's charging time to under five minutes. Photo courtesy Purdue University
WEST LAFAYETTE, IN—Engineers at Purdue University have invented a new charging station cable that can fully recharge some electric vehicles in less than five minutes. That’s approximately the same amount of time it currently takes to fill up a gas tank.
Today, chargers are limited in how quickly they can charge an EV’s battery due to the danger of overheating. To charge an EV faster, a higher current needs to travel through the charging cable. The higher the current, the greater amount of heat that must be removed to keep the charging cable operational. The cooling systems that chargers currently use remove only so much heat.
Using an alternative cooling method, Purdue engineers designed a charging cable that can deliver a current 4.6 times that of the fastest available EV chargers on the market today by removing up to 24.22 kilowatts of heat. The research effort was funded by Ford Motor Co.
“Electric vehicle charging time can vary widely today, from 20 minutes at a station alongside a roadway to hours using an at-home charging station,” says Issam Mudawar, Ph.D., a mechanical engineering professor at Purdue. “Wait times and charger location are both cited as major sources of anxiety for people who are considering electric vehicle ownership."
Though the prototype hasn’t been tested on EVs yet, Mudawar and his students demonstrated in the lab that their prototype accommodates a current of over 2,400 amperes—far beyond the 1,400-ampere minimum that would be needed to reduce charging times for large commercial EVs to five minutes. Currently, the most advanced chargers in the industry deliver only currents up to 520 amperes, and most chargers available to consumers support currents of less than 150 amperes.
“Ultimately, charge times will be dependent on the power output ratings of the power supply and charging cable, and the power input rating of the EV’s battery,” explains Mudawar. “To obtain a sub-five minute charge, all three components will need to be rated to 2,500 amperes.”
The prototype mimics all the traits of a real-world charging station. It includes a pump, a tube with the same diameter as an actual charging cable, and the same controls and instrumentation. It also has the same flow rates and temperatures.
EV charging stations and other types of electronics rely on liquid cooling systems to remove heat from within their wires. Increasing the current through a charging cable using this method would require larger conductive wires and more liquid coolant, making the cable heavier and difficult for customers to handle.
Mudawar has spent more than 30 years developing ways to more efficiently cool electronics by taking advantage of how liquid captures heat when boiled into a vapor. By capturing heat in both liquid and vapor forms, a liquid-to-vapor cooling system can remove at least 10 times more heat than pure liquid cooling. These cooling benefits make it possible to use a smaller wire diameter inside the charging cable while dissipating a higher current.
Mudawar and his colleagues plan to work with automakers and charging station manufacturers to test the prototype on EVs within the next two years. The testing will determine more details on charge speeds for specific vehicle models.
“The industry doesn’t really need EVs to charge faster than five minutes, but we think we can increase the current even more by modifying both the state of the incoming liquid and the design of the cooling space around the conductor wires in the charging cable,” says Mudawar.
Aptera Motors Moves Into California Assembly Plant

Aptera Motors plans to assemble its three-wheeled solar-powered EV at a new factory in Carlsbad, CA. Photo courtesy Aptera Motors
CARLSBAD, CA—Aptera Motors Corp. recently moved into its new assembly plant here. The 77,147-square-foot facility will be the first of several microfactories that the company plans to build around the world to produce its three-wheeled solar vehicle.
“We’ll start by producing our first few hundred vehicles, the Paradigm Edition,” says Pablo Ucar, vice president of production and procurement at Aptera Motors. “As our supply chain becomes more established, we’ll ramp to 250 per month, and eventually reach our target of producing 40 vehicles per day.”
“Over the coming months, we will be completing our beta vehicles, validating our production parts, and moving into testing and certification,” adds Chris Anthony, CEO of Aptera Motors. “We have over 13,000 customers on our reservation list and our engineering team is working around the clock with a goal to begin delivering vehicles in 2022.”
The vehicle’s carbon-fiber composite body will be covered in 3 square meters of solar cells. According to Anthony, at least 90 percent of the power produced by the solar panels will go toward propelling the vehicle. Boasting 100 watt hours per mile, he claims it will be “the most efficient vehicle on the planet.”
“Integrated solar can be configured to provide up to 45 miles of range per day,” explains Anthony. “[This will be the] first vehicle capable of meeting most daily driving needs using solar power alone.”
Zimmer Showcases EV Battery Grippers at the Assembly Show

The Zimmer booth at the recent Assembly Show featured a Tesla Model 3 sedan. Photo by Austin Weber
ROSEMONT, IL—Zimmer Group US Inc. showcased its innovative robotic gripper technology at the recent Assembly Show here with a special booth that featured a Tesla Model 3 sedan. It also displayed a six-axis high-speed palletizing robot from ABB Robotics lifting a 20-kilogram battery module.
“Tesla is a good customer of ours,” says Russell Tyler, sales manager for system technology at Zimmer Group. “They use our grippers for assembling battery modules, handling battery packs and unloading aluminum die-castings.
“Many people who stopped by our booth asked why we had the car on display,” says Tyler. “It was a good way to create some brand awareness and explain our participation in the electric vehicle industry, which is a big growth area for us. In addition to EV battery handling applications, our grippers are used in electric motor assembly, especially when it comes to handling rotors and stators.
“While EV applications certainly are popular today, we’ve been involved in the auto industry for a long time,” Tyler points out. “Our products are routinely used throughout the sector to handle heavy parts such as engine blocks, tires, wheels and windshields. And, batteries aren’t even the heaviest item they’re used with; we’ve been involved in applications where the parts weigh up to 2 tons.
“The gripper that we had on display at the Assembly Show was a custom unit, but it incorporated our standard tool changer and our GPH8000 Series gripper,” says Tyler. “The GPH8000 is a two-jaw, parallel, long-stroke gripper that features up to 150-millimeter stroke per jaw. It’s designed for picking up wide, heavy parts.”
German Team Wins Autonomous Indy Car Race
INDIANAPOLIS—A team of students from the Technical University of Munich (TUM) won the Indy Autonomous Challenge (IAC) here. They competed in a field of nine teams from 21 universities to win the $1 million grand prize at the Indianapolis Motor Speedway.
The rules of the IAC competition required each team to compete in a fastest lap competition that included an obstacle avoidance component. The winning team recorded the fastest two-lap average speed of 135.944 mph on the world-famous 2.5-mile oval race track.
“Participating in the Indy Autonomous Challenge allowed our team to advance autonomous driving technology, and being able to take first place after two years of hard work acknowledges that we had an outstanding team,” says Alex Wischnewski, team leader of TUM Autonomous Motorsport. “Our next goal is to win a high-speed autonomous head-to-head race.”
According to Wischnewski, the prize money will help support TUM’s efforts to further autonomous technology research and development.
A variety of automotive and high-tech suppliers were involved in the IAC, including Ansys, Bridgestone, Cisco, Dallara, Hexagon, Intel, Microsoft and Schaeffler.
Prior to the on-track time trials, a package delivery drone from Telegrid Technologies Inc. dropped off a box containing a checkered flag. Boston Dynamics Inc.’s Spot mobile robot served as the official IAC flag waver for the event.
The next round of the Indy Autonomous Challenge will be held on Jan. 7, 2022, at the Las Vegas Motor Speedway.
Changes in Production and Materials Can Impact EV Battery Life

Prototype lithium-ion batteries undergo diagnostic measurements inside an environmentally controlled chamber. Photo courtesy University of Michigan
ANN ARBOR, MI—Engineers at the University of Michigan have developed a method for predicting how changes to manufacturing processes and materials can impact battery life. They have discovered that internal resistance, measured immediately after cells are made, are a key indicator of how long a battery will last. The measurements can be done in just seconds at the tail end of the production process at little to no additional cost.
Previous research has shown lifespan prediction is possible, but it requires repeated cycling—charging, discharging and recharging—to gather data needed to train the algorithm. Aging tests needed to determine lifespan can also take weeks to months to complete. For this reason, the tests are typically performed on a limited basis.
But, accurate battery lifetimes can be predicted with the help of one resistance measurement, or a measure of how much the battery fights the flow of current inside it. That resistance can come from the materials used for internal components, or electrochemical factors that affect how well ions move between the battery electrodes.
Measuring resistance at low levels of charge is the key. The resistance measurement, at low state of charge, can, in principle, be obtained without any cycling, making the model training process much faster.
“It can give an indication of how much lithium merged with the liquid electrolyte that ferries ions from one electrode to the other inside the battery,” says Anna Stefanopoulou, Ph.D., a professor of technology who is leading the research effort at the University of Michigan Battery Lab. “That combination layer, known as the solid electrolyte interphase, can protect the surface of the electrode and enable longer lifetimes.
“The amount of lithium that goes into the solid electrolyte interphase is usually difficult to measure,” notes Stefanopoulou. “But, at a low state of charge, the battery’s internal resistance is closely related to how much lithium went into the solid electrolyte interphase. This provides a quick measure of that protective layer, as well as the operating capacity of the battery, with ordinary equipment.
“Automakers are always trying to decrease the cost of producing cars, and right now, they’re looking to make EV batteries as cost-effective as possible,” explains Stefanopoulou. “So the question we’ve tried to answer is ‘How fast can you learn about battery lifetime during the manufacturing process itself?’
“It turns out that the answer is, ‘Immediately, if you know the critical signal that can be acquired in high-throughput testing,’” says Stefanopoulou. “Finding such key measurable features can be used for continuous improvements and scaling up domestic battery manufacturing.”
New Process Makes EV Battery Recycling More Economical
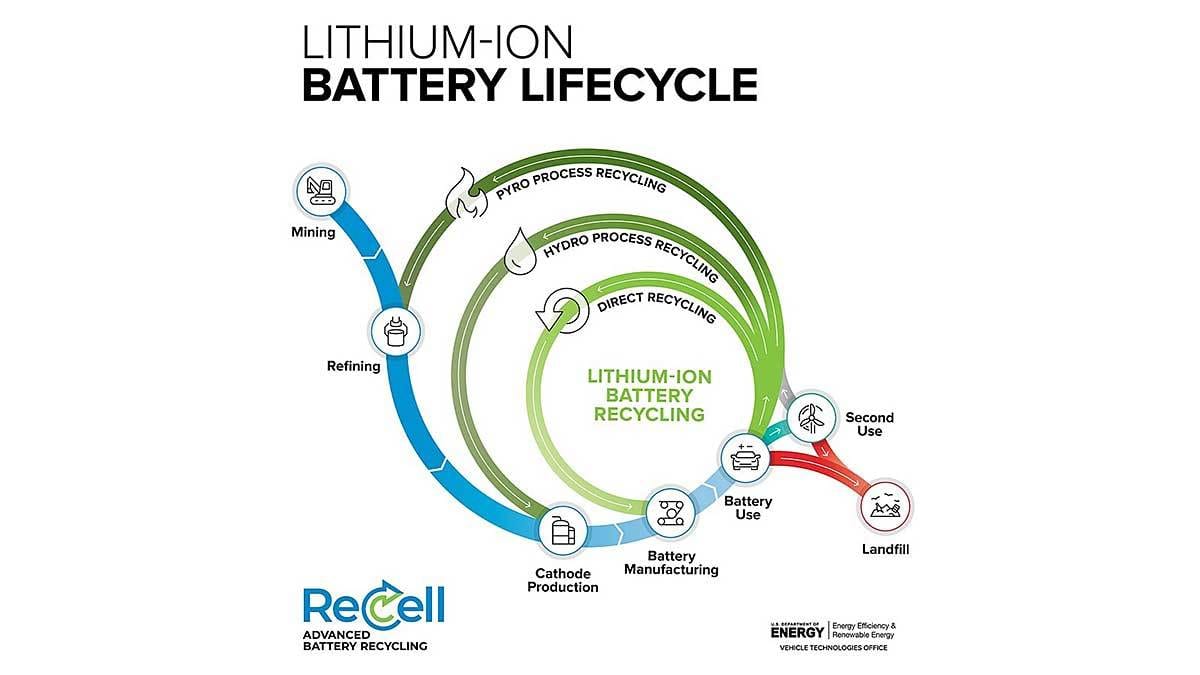
Direct recycling and reusing battery cathode materials closes the loop. Illustration courtesy Argonne National Laboratory
LEMONT, IL—Engineers at Argonne National Laboratory’s ReCell Center have discovered a way to make lithium-ion battery recycling more economically viable. They’re using an innovative process for separating the valuable materials that make up the cathode, a battery’s positively charged electrode.
“The recycling processes being used today enable the recovery of metals in forms that are of low value to battery manufacturers,” says Jessica Durham, a materials scientist at Argonne working on the project, which involves scaling up a separation process originally developed at Michigan Technological University.
“An enormous problem looms on the horizon,” warns Durham. “In less than a decade, [projections indicate] that 2 million tons of end-of-life lithium-ion batteries from EVs will be retired each year. The number of end-of-life EV batteries is currently low, but it’s about to rise substantially as older model vehicles reach the end of their useful life, and the current recycling infrastructure is not ready for the influx.
“If the battery industry is going to buy recycled cathode material to reuse in new batteries, [it is] not going to sacrifice purity,” Durham points out. “Because the cathode materials of EV batteries vary depending on the automaker and the production year, a recycler must take a mixture of lithium metal oxides, such as lithium cobalt oxide, lithium nickel manganese cobalt oxide, lithium nickel cobalt aluminum oxide and lithium iron phosphate, and separate out each in order for those materials to be reused.”
According to Durham, that once impossible task suddenly seems feasible, thanks to a new twist on an old process called froth flotation. Used for many years by the mining industry to separate and purify ores, froth flotation separates materials in a flotation tank based on whether they repel water and float, or absorb water and sink.
Generally, cathode materials sink, which makes them difficult to separate from each other. That’s true of lithium nickel manganese cobalt oxide (NMC111) and lithium manganese oxide, two common EV battery cathode materials that the ReCell team used in its experiments.
What the engineers found was that separation can be achieved by making one of the cathode materials, NMC111, float via the introduction of a chemical that makes the target material repel water.
Once the cathode materials were separated, they determined through testing that the process had a negligible impact on the electrochemical performance of the materials. Both also had high purity levels (95 percent or above).
“That’s very important,” says Durham. “[This] promises to have wide-ranging implications, such as reducing the cost of recycling lithium-ion batteries; spurring the growth of a profitable recycling market for end-of-life lithium-ion batteries; driving down the cost of EVs for both producers and consumers; enabling the United States to compete in the global battery recycling industry; strengthening U.S. energy independence by increasing the use of domestic sources of recycled battery materials; and reducing U.S. dependence on foreign sources of materials.”
But, for now, Durham and her colleagues are focused on creating, step by step, a complete recycling process for lithium-ion batteries that is economically viable.
“Whatever method is used to do this recycling, the recycler has to be able to profit from it,” says Durham. “We’re putting the steps together knowing that, in the end, the total process is going to have to be profitable.”
Nuro to Build AV's in Las Vegas

Nuro plans to assemble its autonomous delivery vehicles at a new factory in Las Vegas. Photo courtesy Nuro Inc.
LAS VEGAS—Autonomous vehicle manufacturer Nuro Inc. is investing $40 million in a state-of-the-art assembly plant to mass-produce its third-generation delivery vehicle, the R2.
“This is a significant moment for [us],” says Jiajun Zhu, CEO of Nuro. “Building on our tremendous momentum—including strategic partnerships with industry leaders such as Domino’s, Kroger and FedEx—we are now able to invest in the infrastructure to build tens of thousands of robots.”
Encompassing more than 125,000 square feet of space and 80 acres of property development, including a test track, the new factory will enable Nuro to quickly produce AVs with help from BYD North America.
BYD will deliver an assembled-in-America electric vehicle platform that Nuro will transform into AVs. Nuro plans to design, develop and operate all software and digital infrastructure from United States-based servers to ensure safety and privacy.
As part of the project, Nuro will be taking over 74 acres of the Las Vegas Motor Speedway to build a world-class, closed-course testing facility that will allow engineers to develop autonomous vehicles for on-road applications. The test track will focus on a broad range of scenarios, from avoiding pedestrians and pets to giving bicycles space on shared roadways, as well as environmental tests and vehicle systems validation.
The assembly plant and test track are expected to be fully operational in 2022.
DECember 2021 | ASSEMBLYMAG.com

Scroll to
read full story
