By Austin Weber, Senior Editor // webera@bnpmedia.com
Solid-State Battery Is Long-Lasting and Energy Dense
AV/EV tech trends
The all solid-state battery consists of a cathode composite layer, a sulfide solid electrolyte layer and a carbon free micro-silicon anode (left). A chemical reaction (middle) causes expansion and densification of the micro-silicon particles, forming a dense lithium-silicon alloy electrode (right). Illustration courtesy University of California San Diego
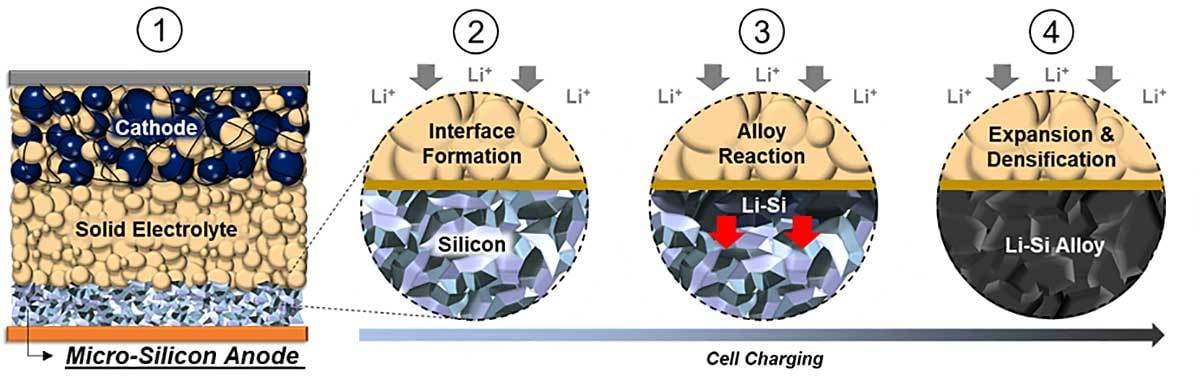
A team of engineers at the University of California San Diego have created a new type of battery that weaves two promising subfields into a single product. They used both a solid-state electrolyte and an all-silicon anode to produce a silicon all-solid-state battery. Initial rounds of tests show that the new battery is safe, long lasting and energy dense.
“With this battery configuration, we are opening a new territory for solid-state batteries using alloy anodes such as silicon,” says Darren Tan, Ph.D., who headed up the project at the Sustainable Materials and Energy Laboratory. Tan worked with LG Energy on the project and recently co-founded a startup company called UNIGRID Battery that has licensed the technology.
“Silicon anodes are famous for their energy density, which is 10 times greater than the graphite anodes most often used in today’s commercial lithium-ion batteries,” explains Tan. “On the other hand, silicon anodes are infamous for how they expand and contract as the battery charges and discharges, and for how they degrade with liquid electrolytes. These challenges have kept all-silicon anodes out of commercial lithium-ion batteries, despite the tantalizing energy density.
“Next-generation, solid-state batteries with high energy densities have always relied on metallic lithium as an anode,” Tan points out. “But, that places restrictions on battery charge rates and the need for elevated temperature (usually 60 C or higher) during charging. The silicon anode overcomes these limitations, allowing much faster charge rates at room-to-low-temperatures, while maintaining high energy densities.”
Tan and his colleagues demonstrated a laboratory-scale full cell that delivers 500 charge and discharge cycles with 80 percent capacity retention at room temperature.
For decades, battery manufacturers have looked to silicon as an energy-dense material to mix into, or completely replace, conventional graphite anodes in lithium-ion batteries. Theoretically, silicon offers approximately 10 times the storage capacity of graphite.
In practice, however, lithium-ion batteries with silicon added to the anode typically suffer from real-world performance issues. In particular, the number of times the battery can be charged and discharged while maintaining performance is not high enough.
“Much of the problem is caused by the interaction between silicon anodes and the liquid electrolytes they have been paired with,” says Tan. “The situation is complicated by large volume expansion of silicon particles during charge and discharge. This results in severe capacity losses over time.”
Tan and his colleagues eliminated the carbon and the binders typically used with all-silicon anodes. In addition, they used micro-silicon, which is less expensive than nano-silicon. In addition to removing all carbon and binders from the anode, the engineers removed the liquid electrolyte. Instead, they used a sulfide-based solid electrolyte.
“The solid-state silicon approach overcomes many limitations in conventional batteries,” claims Tan. “It presents exciting opportunities for us to meet market demands for higher volumetric energy, lowered costs and safer batteries.”
New Technology Gives Autonomous Vehicles ‘X-Ray Vision’

Australian engineers have developed a system that enables autonomous vehicles to track moving pedestrians hidden behind buildings and cyclists obscured by cars, trucks or buses. The technology enables vehicles to break the physical and practical limitations of onboard perception sensors, and embrace improved perception quality and robustness, by using x-ray style vision that penetrates through to pedestrian blind spots.
“This is a game changer for both human-operated and autonomous vehicles which we hope will substantially improve the efficiency and safety of road transportation,” says Eduardo Nebot, Ph.D., professor emeritus at the University of Sydney’s school of aerospace, mechanical and mechatronic engineering. He conducted the research with the Australian Centre for Field Robotics, Cohda Wireless and the iMOVE Cooperative Research Centre.
A new system enables autonomous vehicles to track moving pedestrians hidden behind buildings and cyclists obscured by cars, trucks or buses. Illustration courtesy Cohda Wireless
“Using collective perception, the connected vehicle was able to track a pedestrian visually obstructed by a building,” explains Nebot. “This was achieved seconds before its local perception sensors or the driver could possibly have seen the same pedestrian around the corner, providing extra time for the driver or the navigation stack to react to this safety hazard.”
Using roadside intelligent transportation system (ITS) stations, vehicles can share what they “see” with others using vehicle-to-X (V2X) communication. This system significantly increases the vehicles’ range of perception by allowing them to tap into various viewpoints.
According to Nebot, the technology could benefit all vehicles, not just those connected to such a system. The applications, which are being commercialized by Cohda, involve an emerging ITS technology called cooperative or collective perception (CP).
Another experiment conducted by Nebot and his colleagues demonstrated how collective perception could allow vehicles to safely interact with walking pedestrians, with the vehicle’s response based on the perception information provided by the roadside ITS station. The three-year project also demonstrated the expected behavior of a connected vehicle when interacting with a pedestrian rushing toward a designated crossing area.
“Using the ITS system, the connected autonomous vehicle managed to take preemptive action: braking and stopping before the pedestrian crossing area based on the predicted movement of the pedestrian,” says Nebot.
“The pedestrian tracking, prediction, path planning and decision making were based on the perception information received from the ITS roadside stations,” explains Nebot. “Collective perception enables smart vehicles to break the physical and practical limitations of onboard perception sensors.
“[Our] research confirms that using CP could improve awareness of vulnerable road users and safety in many traffic scenarios,” adds Nebot.
High-rate Magnesium Rechargeable Batteries Move One Step Closer to Reality
Magnesium rechargeable batteries (MRBs), in which high-capacity magnesium is used as the anode material, are promising candidates for next-generation batteries due to their energy density, safety and cost. However, the lack of high-performance cathode materials impedes their development.
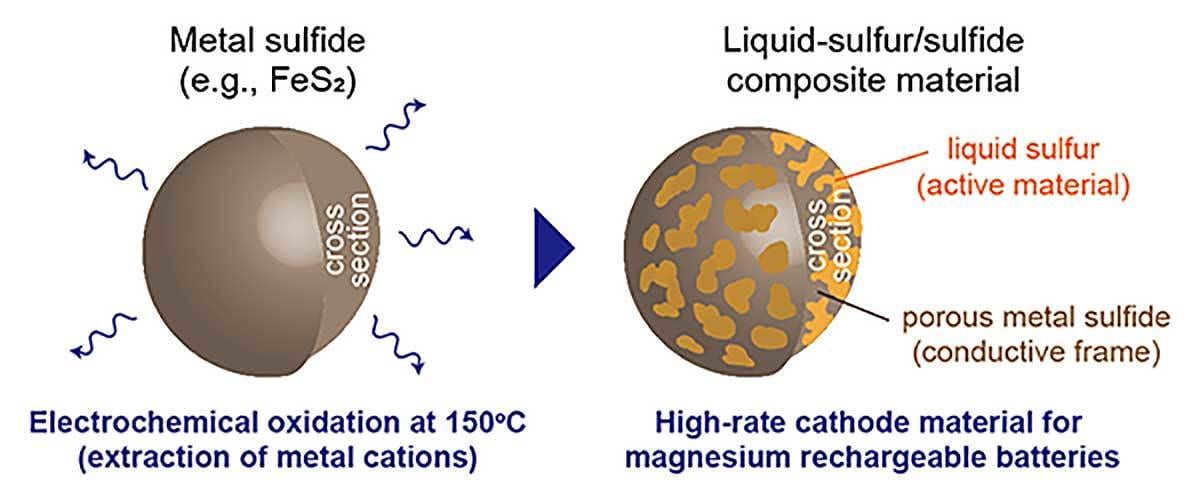
To address the issue, engineers at Tohoku University have developed liquid-sulfur, sulfide-composite cathodes that enable high-rate magnesium batteries. The materials can be spontaneously fabricated by electrochemically oxidizing metal sulfides, such as iron sulfide, in an ionic liquid electrolyte at 150 C. The composite material showed high performance in capacity, potential, cyclability and rate capability.
Liquid-sulfur, sulfide-composite materials fabricated by electrochemical oxidation of metal sulfides can work as high-performance cathode materials for magnesium rechargeable batteries. Illustration courtesy Tohoku University
“Like their lithium-ion counterparts, transition metal oxides are the staple cathode materials in MRBs,” says Kohei Shimokawa, assistant professor of materials and energy at Tohoku University. “Yet the slow diffusion of magnesium ions inside the oxides poses a serious problem. To overcome this, some researchers have explored sulfur-based materials. But, sulfur-based cathodes for MRBs have severe limitations, such as low conductivity.
“Our material allowed for a stable cathode performance for more than 50 cycles,” claims Shimokawa. “Such a high cyclability could be attributed to the high structural reversibility of the liquid state active material and the low solubility of polysulfides into the ionic liquid electrolyte.
Despite the progress, Shimokawa says several problems remain. “We need electrolytes that are compatible with both the cathode and anode materials, because the ionic liquid used in this work passivates the magnesium-metal anode,” he points out. “In the future, [we hope] to develop new electrochemically stable electrolytes to make MRBs more practical for widespread use in next-generation batteries.”
DECember 2021 | ASSEMBLYMAG.com

Scroll to
read full story
