By Austin Weber, Senior Editor // webera@bnpmedia.com
New Process Revitalizes Batteries by Bringing ‘Dead’ Lithium Back to Life
AV/EV tech trends
Charging and discharging a lithium battery test cell causes an island of “dead,” or detached, lithium metal to creep back and forth between electrodes. The movement of lithium ions back and forth through the electrolyte creates areas of negative (blue) and positive (red) charge at the ends of the island, which swap places as the battery charges and discharges. Illustration courtesy SLAC National Accelerator Laboratory
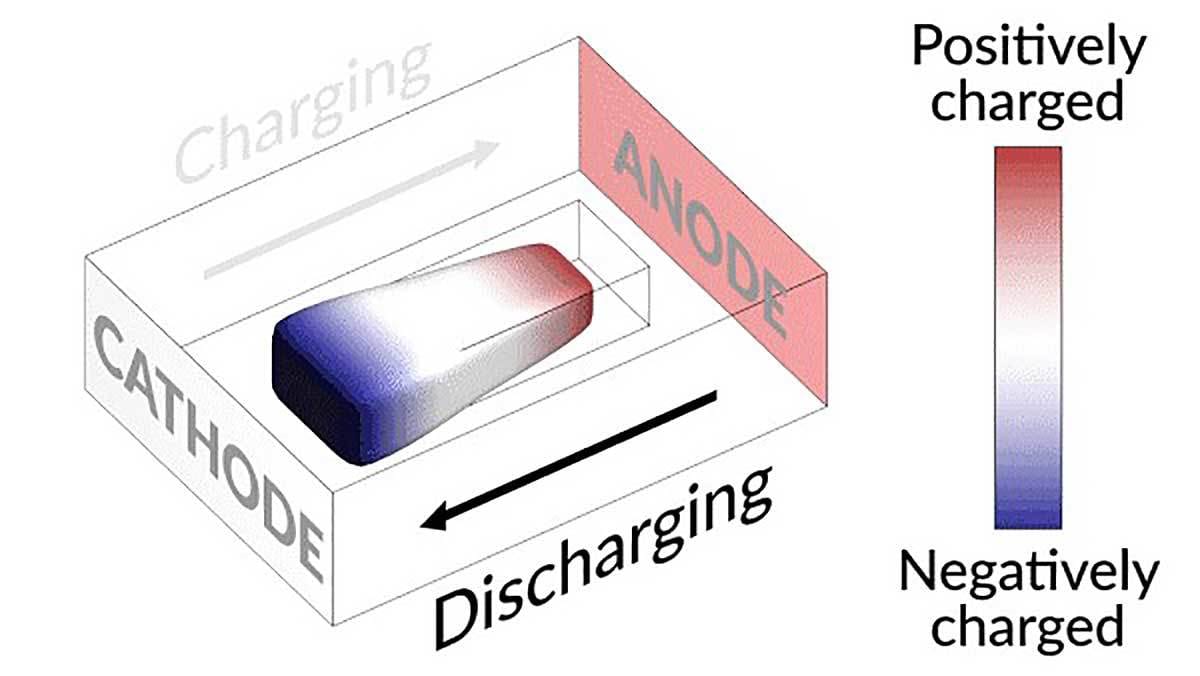
Engineers at the U.S. Department of Energy’s SLAC National Accelerator Laboratory and Stanford University have discovered a way to restore the capacity and lifespan of rechargeable lithium batteries. The breakthrough could potentially help boost the range of electric vehicles.
As lithium batteries cycle, they accumulate little islands of inactive lithium that are cut off from the electrodes, decreasing the battery’s capacity to store charge. The engineers forced this “dead” lithium to creep like a worm toward one of the electrodes until it reconnects, partially reversing the unwanted process. Adding this extra step slowed the degradation of their test battery and increased its lifetime by nearly 30 percent.
Lithium-metal batteries hold promise, because they may be able to store more energy per volume or weight. That could create batteries that feature lighter weight, longer lifetimes, improved safety and faster charging speeds than the lithium-ion technology currently used in EVs. These next-generation batteries would increase mileage per charge and take up less space.
Both types of batteries use positively charged lithium ions that shuttle back and forth between electrodes. Over time, some of the metallic lithium becomes electrochemically inactive, forming isolated islands of lithium that no longer connect with the electrodes. This results in a loss of capacity and is a particular problem for lithium-metal technology and for the fast charging of lithium-ion batteries.
“I always thought of isolated lithium as bad, since it causes batteries to decay and even catch on fire,” says Yi Cui, Ph.D., a materials science professor at Stanford who led the research project. “But, we have discovered how to electrically reconnect this ‘dead’ lithium with the negative electrode to reactivate it.”
The idea for the study was born when Cui speculated that applying a voltage to a battery’s cathode and anode could make an isolated island of lithium physically move between the electrodes. To confirm it, he fabricated an optical cell with a lithium-nickel-manganese-cobalt-oxide cathode, a lithium anode and an isolated lithium island in between.
Cui and his colleagues discovered that the isolated lithium island wasn’t dead at all, but responded to battery operations. When charging the cell, the island slowly moved toward the cathode; when discharging, it crept in the opposite direction.
“It’s like a very slow worm that inches its head forward and pulls its tail in to move nanometer by nanometer,” explains Cui. “In this case, it transports by dissolving away on one end and depositing material to the other end. If we can keep the lithium worm moving, it will eventually touch the anode and reestablish the electrical connection.”
The engineers discovered that they can move the detached lithium toward the anode during discharging, and that these motions are faster under higher currents. So, they added a fast, high-current discharging step right after the battery charges, which moves the isolated lithium far enough to reconnect it with the anode. This reactivates the lithium.
New Anode Material Could Enable Fast-Charging EV Batteries
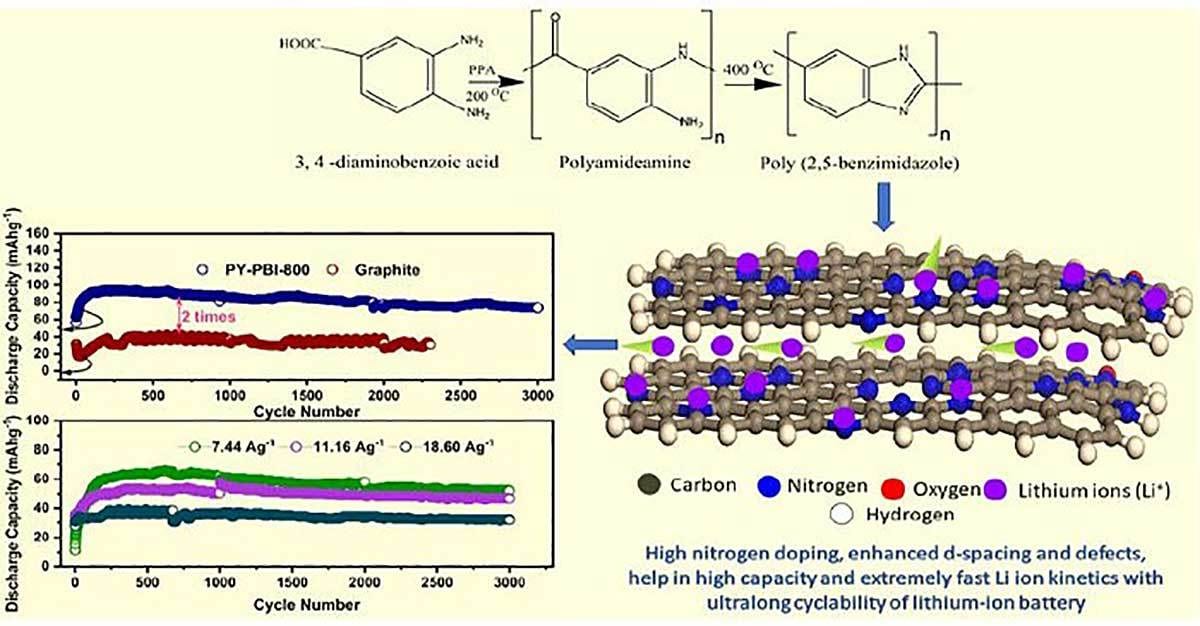
To overcome the slow charging times of conventional lithium-ion batteries, engineers at the Japan Advanced Institute of Science and Technology have developed a new anode material that allows for ultrafast charging. The material, made with bio-based polymers, could enable extremely fast battery charging for electric vehicles.
“Currently, it takes 40 minutes [to charge] EVs, while gas cars can be [refueled] in no longer than five minutes,” says Noriyoshi Matsumi, Ph.D., a materials scientist who headed the research project. “Charging time needs to be below 15 minutes to be a viable option.”
Matsumi and his colleagues used a relatively simple, environmentally sound and highly efficient way to produce a carbon-based anode with very high nitrogen content. The precursor material for the anode is poly (benzimidazole), a bio-based polymer that can be synthesized from raw materials of biological origin.
A new anode material with high nitrogen content and enhanced interlayer spacing allows for faster lithium-ion kinetics both across and between layers. It is very stable and retains most of its original capacity, even after thousands of cycles. Illustration courtesy Japan Advanced Institute of Science and Technology
By calcining this thermally stable material at 800 C, the engineers managed to prepare a carbon anode with a record-setting nitrogen content of 17 percent in weight. They verified the successful synthesis of this material and studied its composition and structural properties using a variety of techniques, including scanning electron tunneling microscopy, Raman spectroscopy and X-ray photoelectron spectroscopy.
To test the performance of their anode and compare it with the more common graphite, the engineers conducted charge-discharge experiments. The results were very promising, as the proposed anode material proved suitable for fast charging, thanks to its enhanced lithium-ion kinetics.
Durability tests showed that a battery with the anode material retained about 90 percent of its initial capacity, even after 3,000 charge-discharge cycles at high rates, which is considerably more than the capacity retained by graphite-based cells.
Another advantage of the new anode material is the use of a bio-based polymer in its synthesis. As a low-carbon technology, the material naturally leads to a synergistic effect that reduces CO2 emissions further. According to Matsumi, modifications to the structure of the polymer precursor could lead to even better performance.
Sodium-Based Material Yields Stable Alternative to Lithium-Ion
A new sodium-based material developed at the University of Texas at Austin is highly stable and capable of recharging as quickly as a traditional lithium-ion battery. It may pave the way toward delivering more energy than current battery technologies.
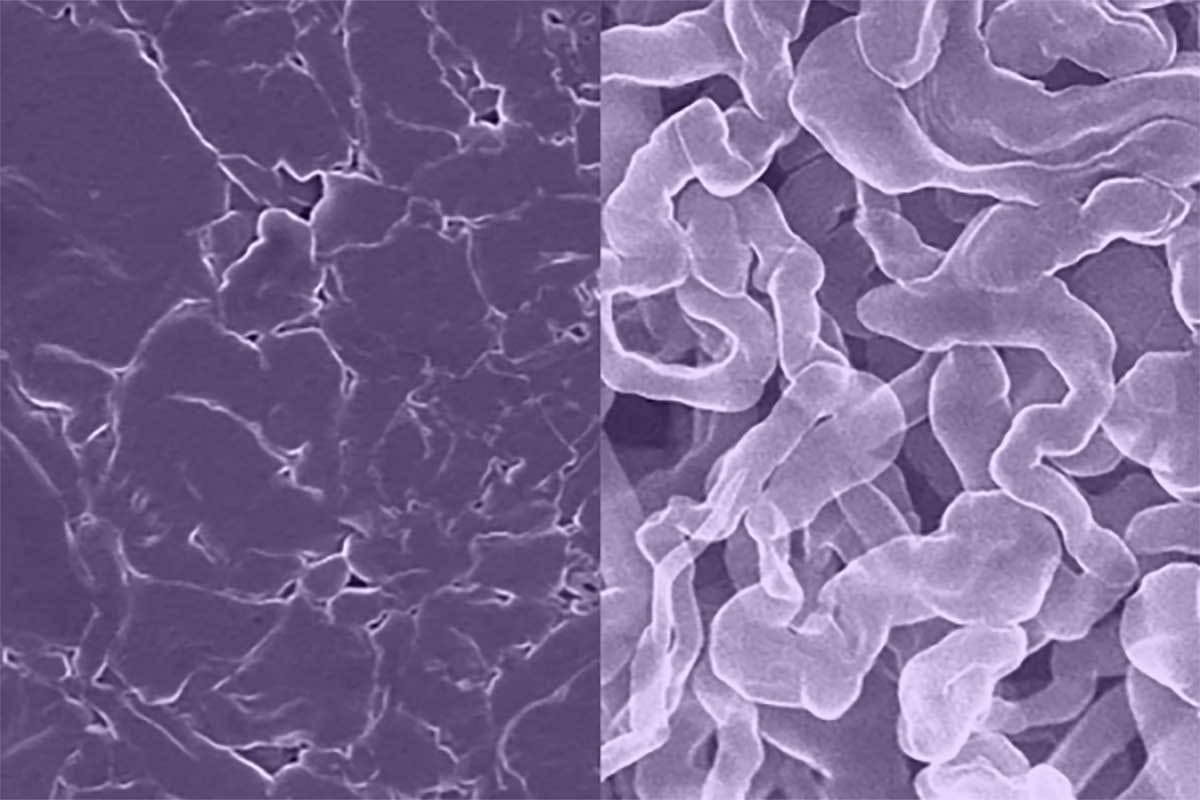
Sodium batteries replace both lithium and cobalt used in current lithium-ion batteries with cheaper, more environmentally friendly sodium. Unfortunately, in earlier sodium batteries, the anode would tend to grow needle-like filaments called dendrites that can cause devices to electrically short and even catch fire or explode.
“We’re essentially solving two problems at once,” says David Mitlin, Ph.D., a mechanical engineering professor who designed the new material. “Typically, the faster you charge, the more of these dendrites you grow. So, if you suppress dendrite growth, you can charge and discharge faster, because all of a sudden it’s safe.”
A new sodium-metal anode for rechargeable batteries (left) resists the formation of dendrites, a common problem with standard sodium-metal anodes (right) that can lead to shorting and fires. Photo courtesy University of Texas at Austin
According to Mitlin, lithium mining has been criticized for its environmental impacts, including heavy groundwater use, soil and water pollution, and carbon emissions. Lithium-ion batteries typically also use cobalt, which is expensive and mined mostly in the Democratic Republic of Congo, where it has significant impacts on human health and the environment. By comparison, sodium mining is cheaper and more environmentally friendly.
The new anode material, called sodium antimony telluride intermetallic (Na metal composite), is made by rolling a thin sheet of sodium metal onto an antimony telluride powder, folding it over on itself, then repeating the process many times.
“Think of making a kind of layered pastry, like spanakopita,” says Mitlin. “This process results in a very uniform distribution of sodium atoms that makes it less likely to form dendrites or surface corrosion than existing sodium-metal anodes. That makes the battery more stable and allows faster charging, comparable to a lithium-ion battery’s charge rate. It also has a higher energy capacity than existing sodium-ion batteries.”
