By Austin Weber, Senior Editor // webera@bnpmedia.com
Micro Supercapacitors Could Boost Battery Performance
AV/EV tech trends
Micro supercapacitors could increase the lifespan of batteries and reduce their charging time in electric vehicles and portable electronic devices. The image on the right depicts a two-inch wide silicon wafer with integrated micro supercapacitors. Illustration courtesy Chalmers University of Technology

A new breed of micro supercapacitors could increase the lifespan of batteries and enable extremely fast charging. Engineers at Chalmers University of Technology have developed a new way to cost effectively mass-produce the devices.
Supercapacitors consist of two electrical conductors separated by an insulating layer. They can store electrical energy and have many positive properties compared to a normal battery. Benefits include much more rapid charging, more efficient energy distribution, and a much greater lifespan without loss of performance with regards to the charge and discharge cycle.
A supercapacitor can extend battery life up to four times in commercial electric vehicles. However, manufacturing the devices has traditionally been a hurdle.
“Today's supercapacitors are too large for many applications where they could be useful,” claims Agin Vyas, a doctoral student in the department of microtechnology and nanoscience at Chalmers University studying the technology. “They need to be about the same size as the battery they are connected to, which is an obstacle to integrating them in mobile phones or electric cars.”
Vyas and his colleagues have been working on new ways to make supercapacitors much smaller. Their micro supercapacitors are so small that they can fit on the circuit boards that control various functions in electric motors and other devices.
“[The system on a chip] units need to be manufactured in such a way that they become compatible with other components in a system circuit and can easily be tailored for different areas of use,” says Vyas. “[In our manufacturing process], micro supercapacitors are integrated with the most common way of manufacturing system circuits: complementary metal-oxide semiconductor (CMOS) technology.
“We use a method known as spin coating, a cornerstone technique in many manufacturing processes,” explains Vyas. “This allows us to choose different electrode materials. We also use alkylamine chains in reduced graphene oxide, to show how that leads to a higher charging and storage capacity.
“Our method is scalable and would involve reduced costs for the manufacturing process,” Vyas points out. “It represents a great step forward in production technology and an important step toward the practical application of micro supercapacitors.
“A method has also been developed for producing micro supercapacitors of up to 10 different materials in one unified manufacturing process, which means that properties can be easily tailored to suit several different end applications,” adds Vyas.
some objects May Be Closer Than They Appear to driverless car sensors
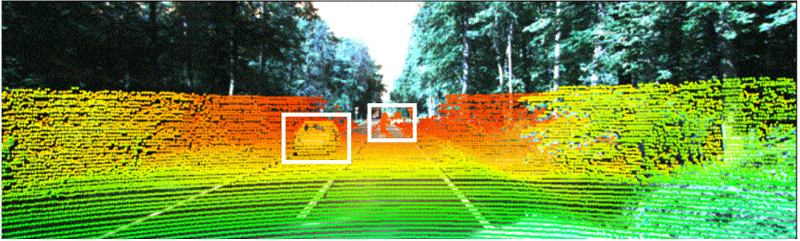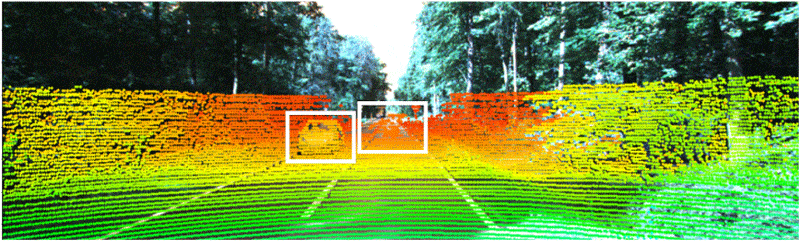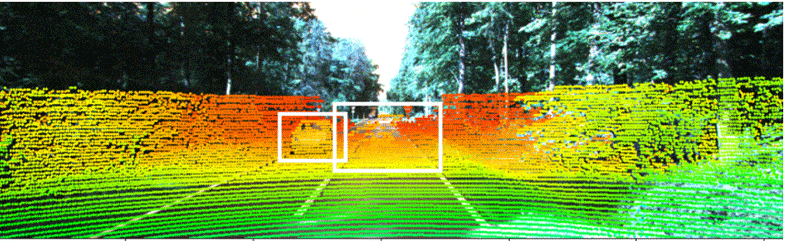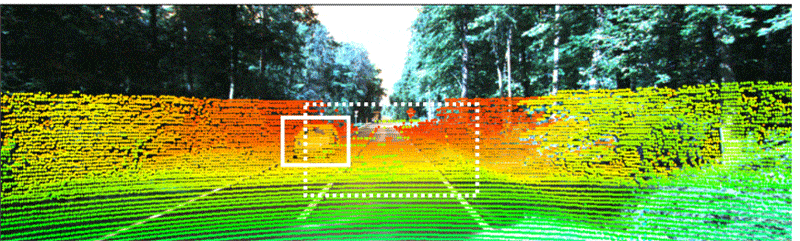
Industry-standard autonomous vehicle sensors can be fooled into believing nearby objects are closer (or further) than they appear without being detected. Adding optical 3D capabilities or the ability to share data with nearby cars may be necessary to fully protect AVs from collisions and cyberattacks. Illustration courtesy Duke University
Industry-standard dual-camera lidar sensors that enable autonomous vehicles to navigate can be deceiving, warn engineers at Duke University. They recently conducted a study that discovered how a popular method to secure sensors against "naive attacks" is still vulnerable at longer distances and only works at short distances.
The project demonstrated why industry-standard AV sensors can be fooled into believing nearby objects are closer (or further) than they appear without being detected. Adding optical 3D capabilities or the ability to share data with nearby cars may be necessary to fully protect driverless cars from collisions and cyberattacks.
A common strategy to secure safety is to check data from separate instruments against one another to make sure their measurements make sense together. Locating technology typically combines 2D data from cameras and 3D data from lidar, which is essentially laser-based radar. This combination has proven very robust against a wide range of attacks that attempt to fool the visual system into seeing the world incorrectly.
“Our goal is to understand the limitations of existing systems so that we can protect against attacks,” says Miroslav Pajic, Ph.D., an associate professor of electrical and computer engineering at Duke University. “This research shows how adding just a few data points in the 3D point cloud ahead or behind of where an object actually is can confuse these systems into making dangerous decisions.”
The new attack strategy works by shooting a laser gun into a car’s lidar sensor to add false data points to its perception. If those data points are wildly out of place with what a car’s camera is seeing, previous research has shown that the system can recognize the attack. But, Pajic and his colleagues have discovered that 3D lidar data points carefully placed within a certain area of a camera’s 2D field of view can fool the system.
This vulnerable area stretches out in front of a camera’s lens in the shape of a frustum—a 3D pyramid with its tip sliced off. In the case of a forward-facing camera mounted on a car, this means that a few data points placed in front of or behind another nearby car can shift the system’s perception of it by several meters.
“This so-called frustum attack can fool adaptive cruise control into thinking a vehicle is slowing down or speeding up,” warns Pajic. “And, by the time the system can figure out there’s an issue, there will be no way to avoid hitting the car without aggressive maneuvers that could create even more problems.”
According to Pajic, there is not much risk of somebody taking the time to set up lasers on a car or roadside object to trick individual vehicles passing by on the highway. That risk increases tremendously, however, in military situations where single vehicles can be very high-value targets. And, if hackers could find a way of creating these false data points virtually instead of requiring physical lasers, many vehicles could be attacked at once.
Pajic claims the best way to protect against these attacks is added redundancy. For example, if cars had “stereo cameras” with overlapping fields of view, they could better estimate distances and notice lidar data that does not match their perception.
“Stereo cameras are more likely to be a reliable consistency check, though no software has been sufficiently validated for how to determine if the lidar and stereo camera data are consistent or what to do if it is found they are inconsistent,” says Spencer Hallyburton, a doctoral candidate in Duke’s Cyber-Physical Systems Lab and the lead author of the study. “Also, perfectly securing the entire vehicle would require multiple sets of stereo cameras around its entire body to provide 100 percent coverage.”
Another option, Pajic suggests, is to develop systems in which cars within close proximity to one another share some of their data. He says physical attacks are not likely to be able to affect many cars at once. And, because different brands of cars may have different operating systems, a cyberattack is not likely to be able to hit all cars with a single blow.
New Electrolyte Improves Solid-State Lithium-Ion Batteries
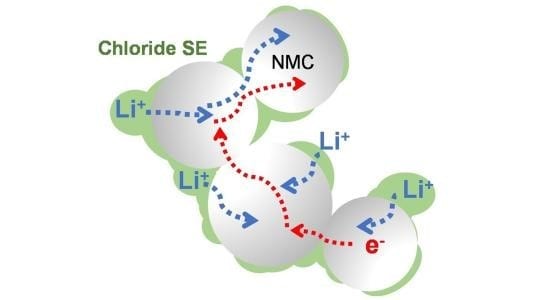
Engineers around the world are scrambling to develop solid-state batteries that can safely store large amounts of energy without using traditional liquid electrolytes. A team at the University of Waterloo (UWaterloo) and the Joint Center for Energy Storage Research (JCESR) at Argonne National Laboratory have discovered a new solid electrolyte that offers several important advantages.
The electrolyte, composed of lithium, scandium, indium and chlorine, conducts lithium ions well but electrons poorly. This combination is essential to creating an all-solid-state battery that functions without significantly losing capacity for more than 100 cycles at high voltage and thousands of cycles at intermediate voltage. The chloride nature of the electrolyte is key to its stability at operating conditions above 4 volts, meaning it’s suitable for typical cathode materials that form the mainstay of today’s lithium-ion cells.
Chlorine-based electrolytes offer improved performance for solid-state lithium-ion batteries. Illustration courtesy University of Waterloo
“The main attraction of a solid-state electrolyte is that it can’t catch fire, and it allows for efficient placement in the battery cell,” says Linda Nazar, Ph.D., a professor of chemistry at UWaterloo and a long-time member of JCESR. “Current iterations of solid-state electrolytes focus heavily on sulfides, which oxidize and degrade above 2.5 volts.
“Therefore, they require the incorporation of an insulating coating around the cathode material that operates above 4 volts, which impairs the ability of electrons and lithium ions to move from the electrolyte and into the cathode,” explains Nazar. “With sulfide electrolytes, you have a kind of conundrum — you want to electronically isolate the electrolyte from the cathode so it doesn’t oxidize, but you still require electronic conductivity in the cathode material.
“Chloride electrolytes have become increasingly attractive, because they oxidize only at high voltages and some are chemically compatible with the best cathodes we have,” Nazar points out. “There’s been a few of them reported recently, but we designed one with distinct advantages.”
One chemical key to the ionic conductivity lay in the material’s crisscrossing 3D structure called a spinel. Nazar and her colleagues had to balance two competing desires—loading the spinel with as many charge carrying ions as possible, but also leaving sites open for the ions to move through.
“You might think of it like trying to a host a dance. You want people to come, but you don’t want it to be too crowded,” says Nazar. “An ideal situation would be to have half the sites in the spinel structure be lithium occupied while the other half remain open. But, creating that situation is hard to design.”
In addition to the good ionic conductivity of the lithium, Nazar and her colleagues needed to make sure that the electrons could not move easily through the electrolyte to trigger its decomposition at high voltage.
“Imagine a game of hopscotch,” explains Nazar. “Even if you’re only trying to hop from the first square to the second square, if you can create a wall that makes it difficult for the electrons, in our case, to jump over, that is another advantage of this solid electrolyte.”
According to Nazar, it’s not yet clear why the electronic conductivity is lower than many previously reported chloride electrolytes. But, it helps establish a clean interface between the cathode material and solid electrolyte—a fact that is largely responsible for the stable performance, even with high amounts of active material in the cathode.
