By Austin Weber // Senior Editor // webera@bnpmedia.com
New Method Tackles Lithium-Ion Battery Recycling
AV/EV tech trends
A new recycling process extracts purified active materials from lithium-ion battery waste. Photo courtesy Rice University

Engineers at Rice University have developed a new method to extract purified active materials from lithium-ion battery waste.
“With the surge in battery use, particularly in EVs, the need for developing sustainable recycling methods is pressing,” says James Tour, Ph.D., professor of materials science and nanoengineering at Rice University.
According to Tour, traditional recycling techniques typically involve breaking down battery materials into their elemental forms through energy-intensive thermal or chemical processes that are costly and have significant environmental impacts.
Tour and his colleagues discovered that magnetic properties can facilitate the separation and purification of spent battery materials. They use a method known as solvent-free flash Joule heating (FJH). This technique involves passing a current through a moderately resistive material to rapidly heat and transform it into other substances.
Using FJH, the Rice engineers heated battery waste to 2,500 Kelvin within seconds, creating unique features with magnetic shells and stable core structures. The magnetic separation allowed for efficient purification.
During the process, cobalt-based battery cathodes—commonly used in EVs and associated with high financial, environmental and social costs—unexpectedly showed magnetism in the outer spinel cobalt oxide layers, allowing for easy separation. This approach resulted in a high battery metal recovery yield of 98 percent, with the value of battery structure maintained.
“Notably, the metal impurities were significantly reduced after separation, while preserving the structure and functionality of the materials,” explains Tour. “The bulk structure of battery materials remains stable and is ready to be reconstituted into new cathodes.”
High-Power Charging Could Ease Range Anxiety

The Next-Generation Profiles project is studying high-power charging technology. Photo courtesy National Renewable Energy Laboratory
High-power charging is quickly becoming the new standard for electric vehicles. At power levels of 200 kilowatts and above, it is expected to help drivers spend less time charging, get back on the road faster, travel longer distances and ease range anxiety.
However, creating a widespread network of reliable and interoperable high-power charging stations is challenging. The technology’s enormous power requirements are more complex than charging light-duty electric passenger vehicles at 40 to 50 kilowatts, and it's more dependent on battery conditions.
The U.S. Department of Energy’s Next-Generation Profiles (NextGen Profiles) project is tackling the issue. Engineers at Argonne National Laboratory, Idaho National Laboratory, the National Renewable Energy Laboratory (NREL) and Oak Ridge National Laboratory are working on the R&D project. They’re studying how electric vehicles and EV supply equipment (EVSE) respond to grid disturbances and smart-charge management scenarios, as well as analyzing how electric fleets perform in those situations.
“The data that we’re collecting addresses a lot of the challenges associated with vehicle electrification,” says Keith Davidson, lead principal investigator for the NextGen Profiles project at NREL. “Chief among those is charging reliability and keeping chargers operating consistently.
“Paramount to a seamless EV charging experience is balancing safety, charging performance and EV battery longevity,” explains Davidson. “Within those requirements, the NextGen Profiles project helps identify high-power charging system limitations, the characteristics of high-power charging sessions (profiles) and issues that the power grid will likely encounter.
“Ultimately, the project seeks to develop a knowledge base that will inform high-power charging infrastructure planning, integrate storage and renewables, and ensure that the transition to EVs is reliable and affordable,” Davidson points out.
The NextGen Profiles project is focusing on several issues. The first is capturing EV profiles by collecting data on how vehicles behave while charging. Engineers are examining how diverse charging conditions affect EVs, and how these profiles compare to different vehicle classes and charging types.
“Taken together, the data paints a holistic picture of the performance of current EV technologies and the trajectory of the industry,” says Davidson.
NREL engineers are also exploring EVSE technology. For instance, they are connecting high-power charging systems to EV emulators and running them through charging scenarios. They’re also assessing the entire range of current and voltage outputs that charging systems provide, including grid disturbance and charge management analysis.
In addition, Davidson and his colleagues are focusing on how EVs charge in extreme temperature conditions. Off-nominal temperature tests are a key component of this work, which involves subjecting vehicles to cold and hot temperature extremes. They are charging, quantifying and analyzing the amount of energy available in batteries at specific points in time relative to their total capacity and age.
“Off-nominal grid conditions tests are critical for EVSEs to measure and evaluate voltage, frequency, harmonics, thermal control systems and standard charging communication protocol response,” explains Davidson. “During a cold soak test, EV and EVSE charging characteristics are assessed when it meets our specific cold temperature conditions.”
Iron May Be Key to Cheaper Lithium-Ion Batteries
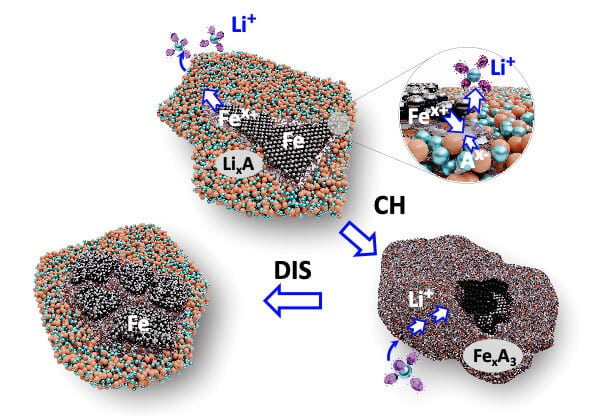
Iron can be used as a cathode material in lithium-ion batteries instead of cobalt and nickel. Illustration courtesy Oregon State University
Engineers at Oregon State University are exploring how iron can be used as a cathode material in lithium-ion batteries instead of cobalt and nickel.
Currently, cathodes represent 50 percent of the cost of making a lithium-ion battery cell. Beyond economics, iron-based cathodes would allow for greater safety and sustainability. Cobalt and nickel supplies are limited. However, iron, in addition to being the most common element on Earth as measured by mass, is the fourth-most abundant element in the Earth’s crust.
The energy density of cobalt and nickel are already being extended to their ceiling levels. If it were pushed further, oxygen released during charging could cause batteries to ignite. Cobalt is also toxic.
“We’ve transformed the reactivity of iron metal, the cheapest metal commodity,” says Xiulei “David” Ji, Ph.D., a professor of chemistry at Oregon State. “Our electrode can offer a higher energy density than the state-of-the-art cathode materials in electric vehicles.
“And, since we use iron, whose costs less than a dollar per kilogram, the cost of our batteries is potentially much lower,” notes Ji. “Our iron-based cathode will not be limited by a shortage of resources.”
Ji and his colleagues increased the reactivity of iron in their cathode by designing a chemical environment that’s based on a blend of fluorine and phosphate anions—ions that are negatively charged.
The blend, thoroughly mixed as a solid solution, allows for the reversible conversion (chargeability) of a fine mixture of iron powder, lithium fluoride and lithium phosphate into iron salts.
“To put this new cathode in applications, one needs to change nothing else—no new anodes, no new production lines and no new design of the battery,” claims Ji. “We are just replacing one thing, the cathode.”
September 2024 | ASSEMBLYMAG.com